Watch our short animated explainer video to understand our technology, below.
OUR SCIENCE
A Smarter Model For Cancer Therapy.
At Jabez, we’re improving cancer therapy in a way that’s both powerful and practical. How? Our “3-S” model guides how we select, advance, and deliver our growing pipeline of cancer therapeutics.
OUR “3-S” THERAPEUTICS MODEL
We identify and advance cancer therapeutics that are easy to take, easy to make, and ready to integrate, not recreate.
“3-S” Cancer Therapeutics are:
Seamless – Easy-to-take
Designed for ease of use; oral administration, well-tolerated profiles, and outpatient compatibility when possible.
Scalable – Easy to make
Synthesized with scalable, tractable chemistry. Built for real-world manufacturing, storage, and distribution.

Synergistic – Ready to combine
Mechanistically synergistic with existing standards of care. We prioritize biological rationale, not empirical stacking.
We’re not redefining biology – we’re leveraging it better.
OUR APPROACH
A Biology-First Approach
We do not rely on trial-and-error combinations. We apply rigorous mechanistic reasoning to identify rationale, biological synergy up front.
Step #1
Identification & Licensing
We identify and license promising molecules with well-characterized mechanisms that align with our 3-S model and strategic synergy criteria.
Step #2
Validation & Planning
We design targeted development plans choosing the precise combinations, and patient populations to maximize impact.
Step #3
Clinical Deployment
We execute focused, efficient trials, bringing the right therapy to the right patients with precision, speed, and intent.
JBZ-001:
Our Lead Compound
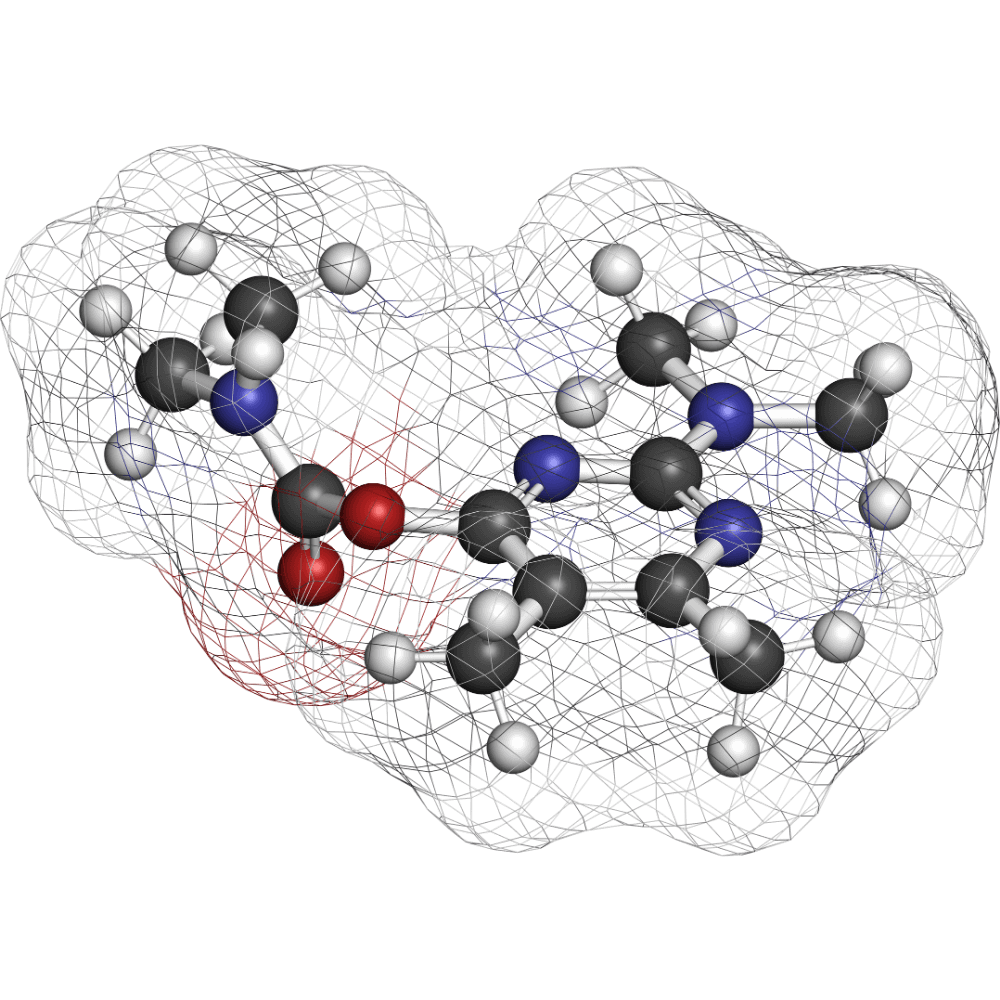
JBZ‑001 is an oral DHODH inhibitor in Phase 1 trials. It’s a proven 3‑S prototype with both single-agent promise and powerful synergy in combination.
It brilliantly exemplifies our 3-S model:
- Seamless: Well-tolerated, oral small molecule
- Scalable: Leverages Nobel Prize-winning, scalable chemistry
- Synergistic: Displays potent synergy with well-established treatment regimens.

JBZ-001: Small Molecule DHODH Inhibitor
DHODH, JBZ-001 & Cancer
To understand how JBZ-001 fights cancer, it is important to first understand the role of DHODH.
DHODH is an enzyme that is crucial for the production of pyrimidine nucleotides – essential factors in the creation of new DNA and RNA, and vital for cell division.
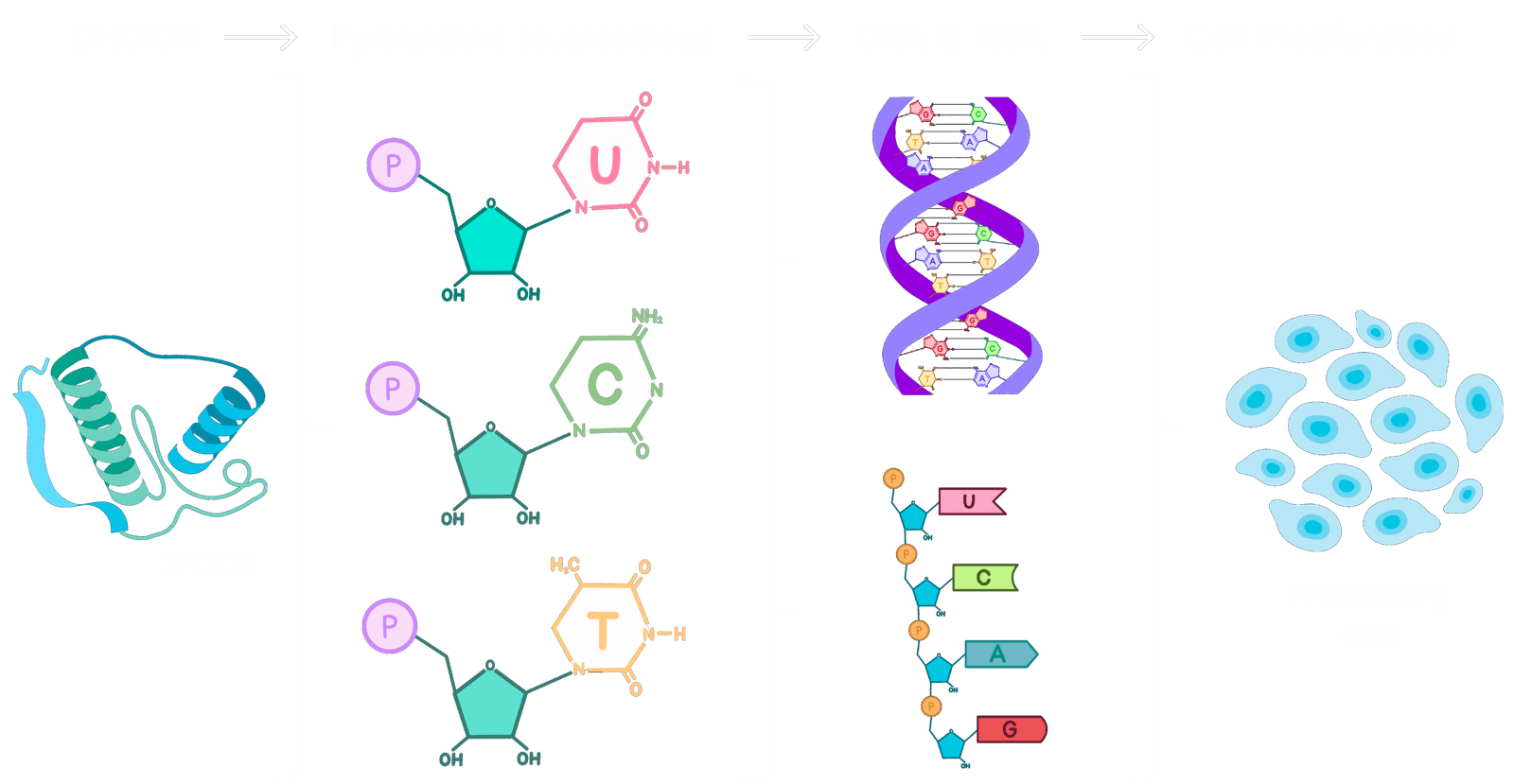
Due to their dysregulated metabolism, cancer cells have a very high demand for nucleotides and therefore are very sensitive to the loss of DHODH function. So, by binding to and inhibiting DHODH, JBZ-001 selectively targets and kills cancer cells.
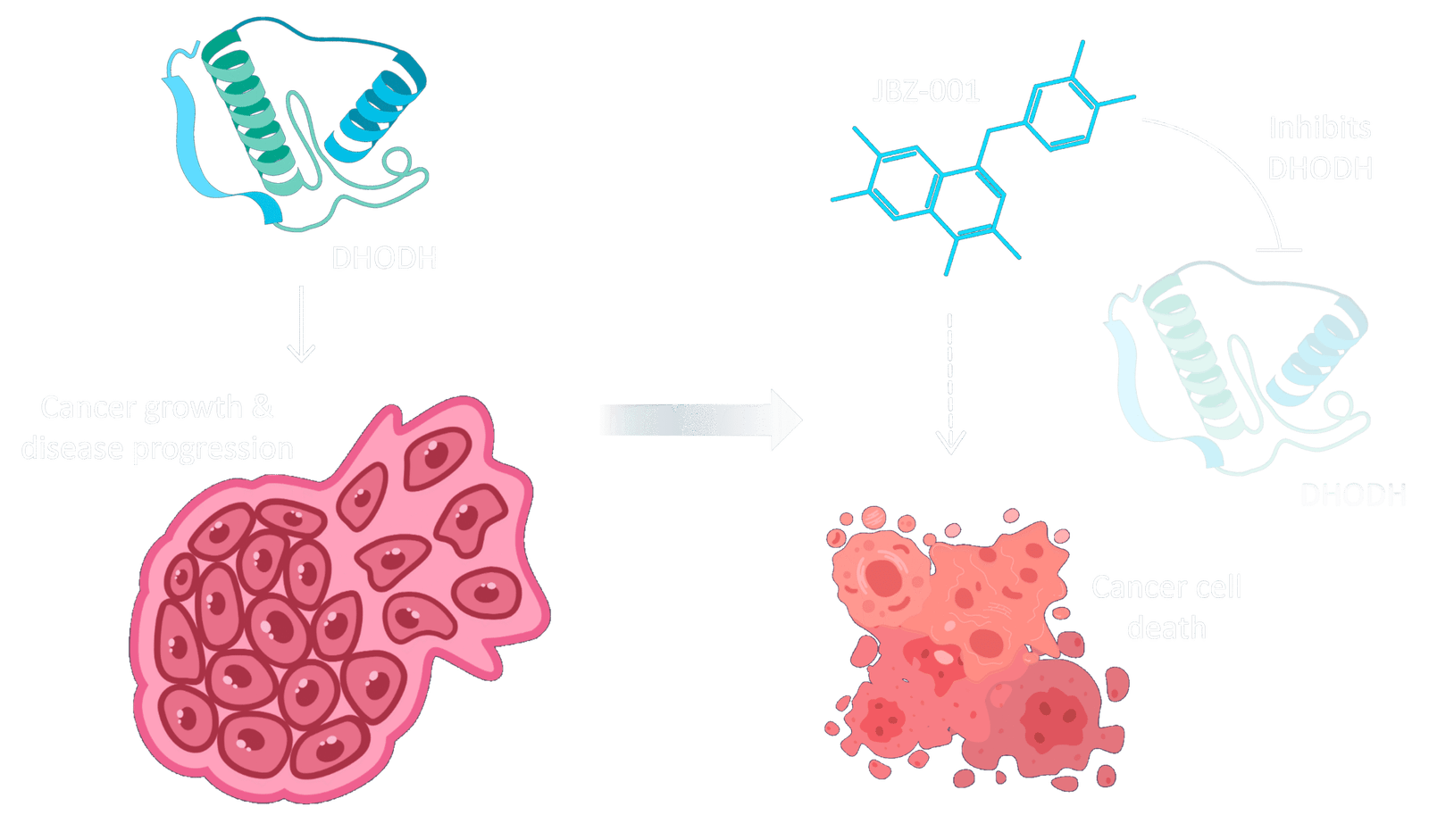
JBZ-001 is Potentially
Best-In-Class.
DHODH inhibition is a well-established therapeutic strategy with decades of clinical precedent.
JBZ-001 builds on that foundation with a highly optimized profile that offers improved potency, tolerability, and oral bioavailability.
Based on its therapeutic properties, we believe JBZ-001 has the potential to be a best-in-class DHODH inhibitor

OUR SYNERGISTIC THERAPEUTIC APPROACH
JBZ-001's Synergistic Potential
While JBZ-001 shows strong single-agent activity, preclinical studies have revealed two remarkable mechanisms of synergy with standard treatments in two key cancer types.
Acute Myeloid Leukemia (AML)
Enhancing AML Treatment
JBZ-001 promotes myeloid cell differentiation and has demonstrated a potent ability to enhance the activity of several AML-specific therapeutics across AML subtypes.
CD38 Antibodies (mAbs)
Increase Activity of CD38 mAbs
In both AML and multiple myeloma models, JBZ-001 increases surface expression of CD38 on tumor cells – dramatically enhancing anti-cancer activity of CD38-targeting antibodies.



